Ribosome-associated Quality Control
Stalled protein synthesis (e.g. due to faulty mRNA) creates defective polypeptides that can be nonfunctional or toxic. Unlike classical protein quality control, which monitors proteins for intrinsic defects such as aberrant 3D structure (“misfolding”), the products of stalled protein synthesis are defective regardless of their intrinsic properties and thus present a challenge for the cell to identify and degrade.
Systematic genetic dissection of the heat shock response in yeast identified a protein complex bound to the large ribosomal subunit and required to ubiquitylate and degrade stalled nascent chains (Brandman et al., 2012). We named this complex the Ribosome-associated Quality Control complex and the process of degrading stalled nascent chains Ribosome-associated Quality Control (RQC) (Brandman and Hegde, 2012). The RQC complex includes Ltn1, Rqc1, Rqc2, the Cdc48-Npl1-Ufd2 subcomplex, and large ribosomal subunits that have split off from stalled ribosomes. Subsequently we characterized genes required for RQC that act upstream of the RQC complex to detect stalled translation, including the identification of three new genes (Slh1, Ykr023w/Rqt4, Cue3) and a gene we previously identified (Hel2) (Sitron et al., 2017). The core complex, consisting of Rqc2 and the large ribosomal subunit, is conserved throughout life (Lytvynenko et al., 2019).
Discovery of the Ribosome-associated Quality Control (RQC) complex
Discovery of a novel modification of stalled nascent chains called “CAT tails”
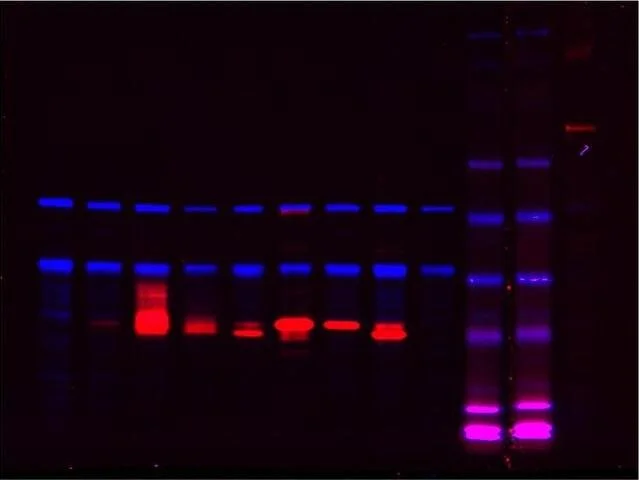
We discovered a novel modification of stalled nascent chains, in addition to classical ubiquitylation. This modification is a C-terminal polypeptide extension of the stalled nascent chain that, remarkably, is not encoded by the stalled mRNA. Chemical analysis revealed that the extensions consist of variable length alanine and threonine amino acids, which we call C-terminal alanine and threonine tails (“CAT tails”) (Shen et al., 2015).
The composition of CAT tails, along with a 3D structure of the RQC complex solved by our collaborators, suggested that CAT tails are generated by a novel form of protein synthesis in which alanine and threonine tRNAs are recruited to the A (aminoacyl) site of the large ribosomal subunit by Rqc2, rather than by anticodon pairing with the mRNA codon as in conventional protein synthesis. The large ribosomal subunit then incorporates the alanine and threonine amino acids on these tRNAs into the nascent polypeptide chain. This mechanism of in vivo protein elongation involves neither mRNA nor the small ribosomal subunit. This remarkable modification to stalled nascent chains (“CAT tails”) demonstrates that the amino acid composition of ribosome-generated proteins in cells is not solely determined by the genetic code (Shen et al., 2015).
CAT tails are generated by a novel form of protein synthesis
CAT tails are degrons targeting stalled nascent chains to the proteasome
Following our discovery of CAT tails, there was an explosion of interest in the function and consequence of this novel protein tag. We discovered that CAT tails function primarily as C-terminal “degrons” that target defective nascent chains for degradation by the proteasome. We found that a secondary function for CAT tails is to enhance ubiquitylation by Ltn1 of structured stalled nascent chains. Thus nascent polypeptide chains stalled during translation are marked by two covalent modifications that target them for destruction: ubiquitin and CAT tails (Sitron and Brandman, 2019).
We discovered that CAT tails drive the heat shock response and thus cause proteotoxic stress (Shen et al., 2015). Consistent with this finding, other labs reported that CAT-tailed proteins form aggregates in cells. We found that aggregation of a CAT-tailed protein is toxic and blocks function of CAT tails as degrons, and alleviating aggregation restores CAT-tail-mediated degradation and reverses toxicity (Sitron et al., 2020). We also discovered that stalled nascent chains that escape RQC accumulate in and damage nucleoli, another form of cell toxicity (Davis et al., 2019). A collaboration with Bingwei Lu’s lab at Stanford found that CAT-tail-like phenomena are conserved in metazoans and linked to neurodegeneration (Wu et al., 2019). Our work may help explain previous studies (Chu et al., 2009) demonstrating that mutations to an RQC gene cause neurodegeneration in mice. The RQC complex and CAT tails are now studied by numerous labs from multiple perspectives, including their mechanism and function across diverse organisms from bacteria to humans, and their roles in toxicity and neurodegenerative diseases. Two reviews from the Brandman Lab cover this emerging field (Brandman and Hegde, 2012; Sitron and Brandman, Annual Review of Biochemistry, 2020, in press).
Pathology of escaped CAT tails
Back to Research